Our Story
Over 18 Years of Excellence
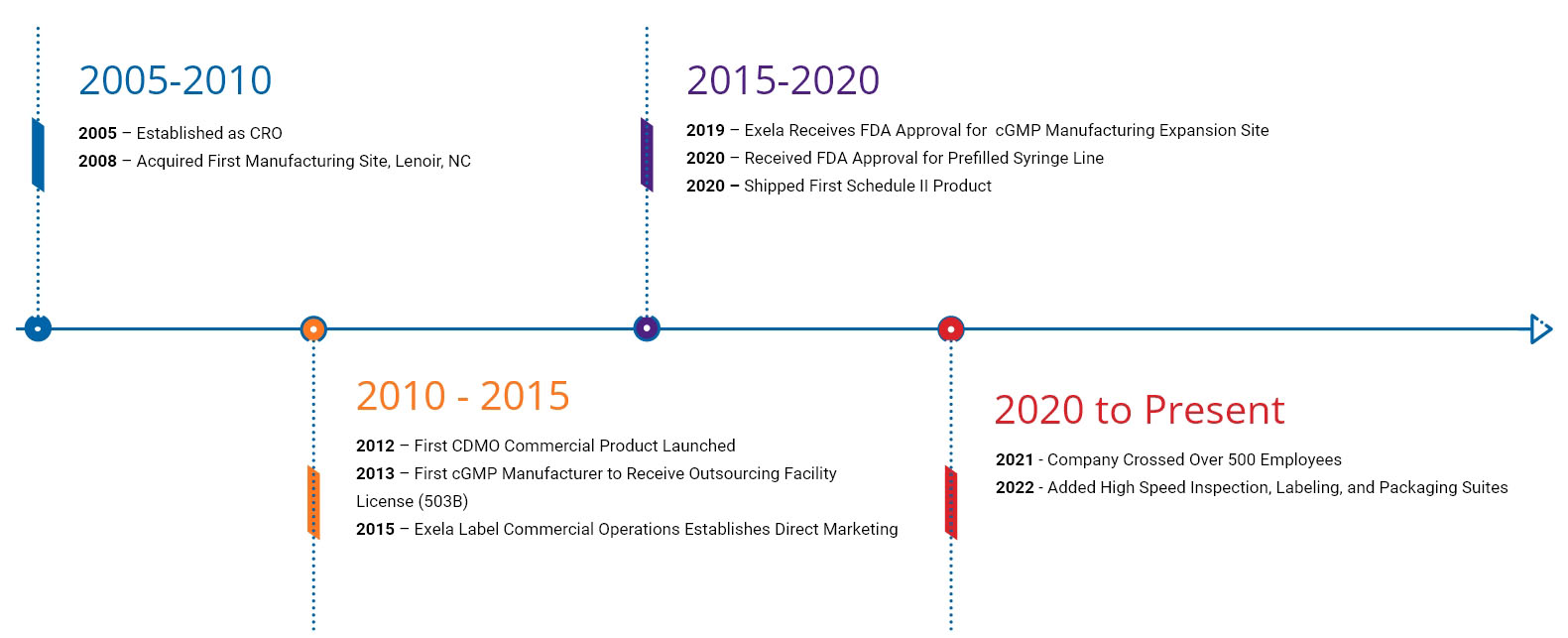
Exela® Pharma Sciences began in 2005 as an R&D organization (CRO) that developed products and partnered those products with leading injectable manufacturers and marketers.
In 2008, Exela acquired its first manufacturing facility in Lenoir, North Carolina. With the addition of manufacturing capabilities, Exela began its transformation from a CRO to a fully functional Contract Development and Manufacturing Organization (CDMO) capable of delivering FDA-approved products in an efficient and timely manner. Our first FDA inspection came in December 2010, and our first FDA product commercial launch occurred in June 2012. Since this humble beginning, Exela has grown its capabilities and footprint in Lenoir to include significant manufacturing, R&D, Quality, warehouse and distribution services.
The FDA approval of our modern manufacturing building in October 2019 expanded our commercial manufacturing capacity significantly.
With the success that came from our CDMO roots, Exela established a commercial organization and launched our first Exela® labeled product in 2015. This new division created the final step in the evolution from a CRO to a fully functional pharmaceutical company specializing in sterile products. Today, in conjunction with our partners, Exela® manufactures and/or markets products in the US, with approval to market in Canada and Australia.

Committed to Our Community

Photo Credit: Joshua Harris, City of Lenoir
