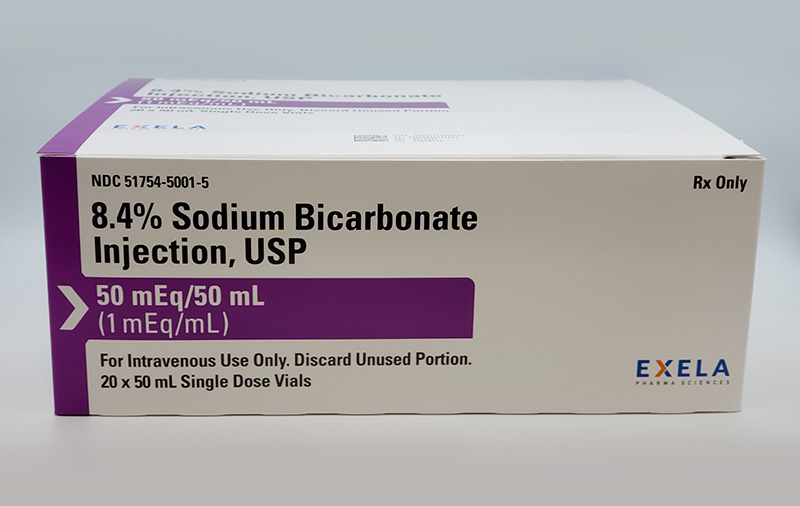
Exela Pharma Sciences, LLC Expands Voluntary Nationwide Recall of Sodium Bicarbonate Injection, USP, 8.4%, 50 mEq/50 mL Vial, 20-Count Carton due to Vial Breakage
UPDATED November 28, 2022
Exela Pharma Sciences, LLC Issues Voluntary Nationwide Recall of Sodium Bicarbonate Injection, USP, 8.4%, 50 mEq/50 mL Vial, 20-Count Carton due to Vial Breakage